Most pitcher plants in the genus Nepenthes seem pretty adept at catching prey. These plants specialize in nutrient-poor soils and their carnivorous habit evolved as a means of supplementing their nutritional needs. Despite the highly evolved nature of their pitfall traps (which are actually modified leaves), Nepenthes aren’t perfect killing machines. In fact, some get a helping hand from seemingly unlikely partners.
Spend enough time reading about Nepenthes in the wild and you will see countless mentions of arthropods hanging around their pitchers. Some of these inevitably become prey, however, there are others that appear to be taking advantage of the plant. Nepenthes don’t passively trap arthropods. Instead, they lure them in with bright colors and the promise of tasty treats like nectar. This is not lost on predators like spiders, who are frequent denizens of pitcher mouths.
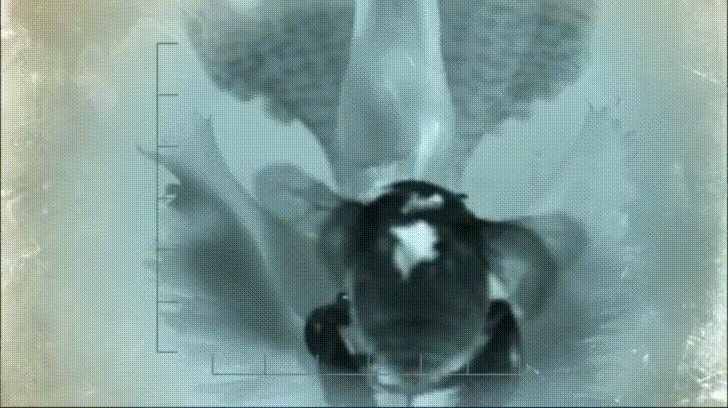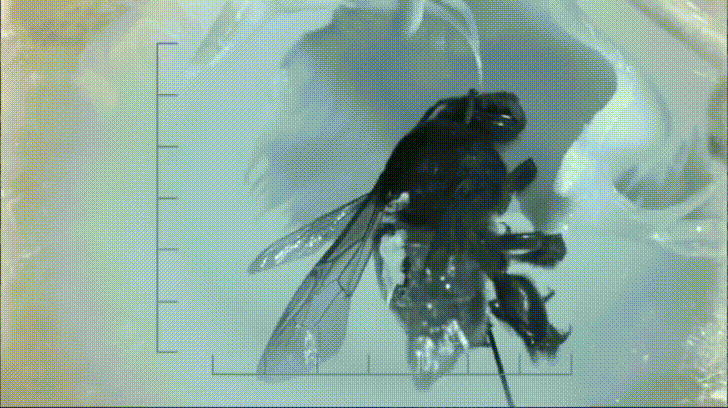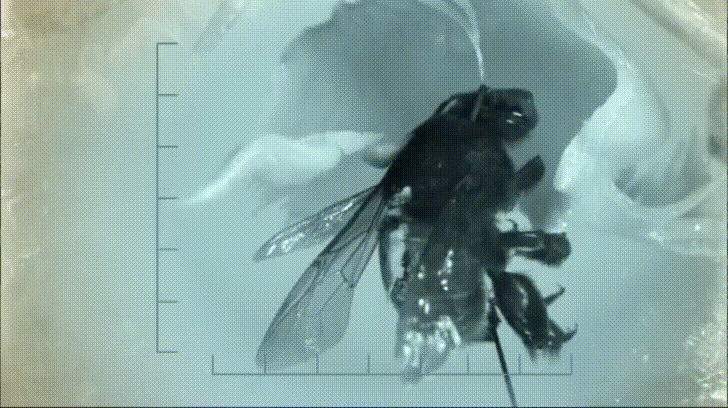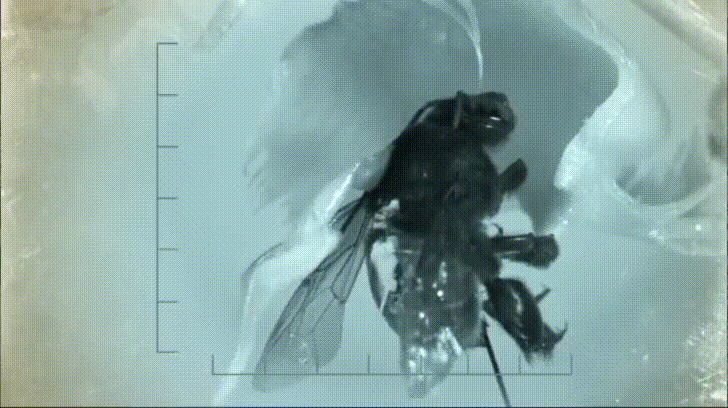
Most notable to Nepenthes specialists are some of the crab spiders that frequently haunt Nepenthes traps. These wonderful arachnids sit at the mouth of the pitcher and ambush any insects that try to pay it a visit. Often times both predator and prey fall down into the pitcher, however, thanks to a strand of silk, the spiders easily climb back out with their meal. This may seem like bad news for the pitcher, however, recent research based out of the National University of Singapore has shown that this relationship is not entirely one sided.
By studying the interactions between spiders and pitcher plants both in the lab and in the field, ecologists discovered that at least one species of pitcher plant (Nepenthes gracilis) appears to benefit greatly from the presence of crab spiders. The key to understanding this relationship lies in the types of prey N. gracilis is able to capture when crab spiders are and are not present.
Not only did the presence of a resident crab spider increase the amount of prey in each Nepenthes pitcher, it also changed the types of insects that were being captured. Crab spiders are ambush predators that frequently attack prey much larger than themselves. It may seem as if this is a form of food robbery on the part of the crab spider but the spiders can’t eat everything. When they have eaten their fill, the spiders discard the carcass into the pitcher where the plant can make quick work digesting it for its own benefit.
Over time, simply having a spider hunting on the trap led to a marked increase in the number of insects in each pitcher compared to those without a spider. Even if these meals are already half eaten, the plant still gains nutrients. Additionally, the types of prey captured by pitchers with and without crab spiders changed. The spiders were able to capture and subdue insects like flesh flies, which normally aren’t captured by Nepenthes pitchers. As such, the resident crab spiders make available a larger suite of potential prey than would be available if they weren’t using the pitchers as hunting grounds.
The crab spiders may benefit the pitcher plant in other ways as well. Research on crab spiders has shown that their bodies are covered in pigments that register high in the UV spectrum. Insects can see UV light and often use it as a means of finding flowers as plants often produce UV-specific pigments in their floral tissues. The wide array of UV patterns on flowers are there to guide their pollinators into position. Researchers have documented that insects are actually more likely to visit flowers with crab spiders than those without, which has led to the idea that UV pigments in crab spiders actually act as insect attractants. Visiting insects simply cannot resist the UV stimulus and quickly fall victim to the resident crab spider.
Could it be that by taking up residence on a Nepenthes pitcher, the crab spiders are increasing the likelihood of insects visiting the traps? This remains to be seen as such questions did not fall under the scope of this investigation. That being said, it certainly offers tantalizing evidence that there is more to the Nepenthes-crab spider relationship. More work is needed to say for sure but the closer we look at such interactions, the more spectacular they become!